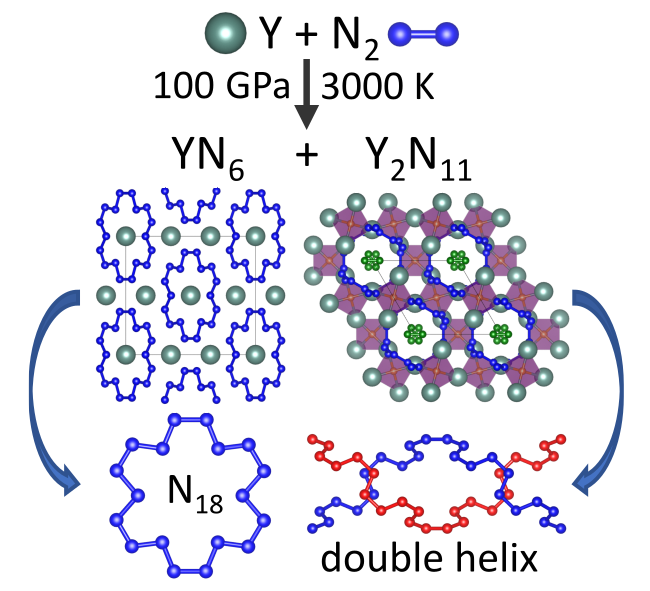
Rare-earth nitrides at high pressure
The chemistry of nitrogen has long been thought to be very limited due to triple-bonded molecular nitrogen's extreme stability. As a result, in inorganic solid-state compounds at ambient pressure, nitrogen is typically present in the form of a nitride anion N3− and does not form catenated polyanions (with an exception of azides). However, over the past 20 years, it has been shown that at high pressure nitrogen’s chemistry significantly changes. For example, charged nitrogen N2x- dimers, tetranitrogen N44- units, pentazolate N5- rings, hexazine N6 rings, and different polynitrogen chains have been synthesized, and an even greater variety of nitrogen species is expected to form under high-pressure conditions according to theoretical calculations. Such a diversity of nitrogen species can suggest that the scale of nitrogen chemistry under high pressure may be close to the scale of the rich carbon chemistry at ambient pressure. In addition to the discoveries of unique nitrogen entities that push the boundaries of fundamental nitrogen chemistry, nitrides and polynitrides synthesized under high pressure often possess key properties for functional applications such as high hardness, unique electronic properties, and high energy density.
Whereas a significant number of studies on binary metal-nitrogen compounds of alkali, alkaline earth, and transition metal elements under high pressure have been conducted, the high-pressure chemistry and physical properties of rare earth metal nitrides are almost unknown. This project is focused on the synthesis of novel rare-earth (poly)nitrides and further exploration of nitrogen high-pressure chemistry.
